Background
High-risk and elderly patients with myeloid neoplasms are a special group because it's challenging to find the right balance between safety and efficacy during the peri-transplant period. Traditional transplant protocols have unsatisfactory treatment outcomes for patients with a high risk of recurrence, while the elderly couldn't tolerate the toxicity of high-dose chemotherapy drugs. Thus, new regimens are needed to improve treatment outcomes for high-risk and elderly patients.
Through BCL-2 binding, venetoclax could induce leukemia cell apoptosis, increase chemosensitivity, and perform a synergistic role with HMAs. And its combination with HMAs or other cytotoxic drugs showed a promising effect on R/R AML. This prospective clinical study reported the efficacy and safety of adding venetoclax and decitabine to myeloablative conditioning regimens in high-risk and elderly patients receiving allogeneic hematopoietic stem cell transplantation.
Methods
We performed an investigator-initiated, prospective, one-arm, single-center, open-label trial. In total, 19 patients were enrolled in the trial between December 2021 and February 2023. Eligible patients met the following inclusion criteria: (1) undergoing allo-HSCT over the age of 50 years (i.e., ≥50 years), (2) having an Eastern Cooperative Oncology Group performance status of 0-2, and (3) having AML, MDS, and CMML with a high risk of recurrence: (a) For patients with MDS, (i) a diagnosis of MDS with excess blasts 2 (EB-2), (ii) a poor karyotype, (iii) a very high-risk disease according to the International Prognostic Scoring System-Revised, and (iv) a TP53 mutation; (b) For patients with AML, (i) relapsed/refractory AML, (ii) detected MRD test before transplantation, (iii) with adverse risk according to the European Leukemia Net (ELN) Guidelines 2017, and (iv) AML transformed from MDS or CMML.
Venetoclax was administered at 400 mg per day orally from day -14 to day -1 in the initial regimen and from day -14 to day -5 in the modified regimen. Decitabine was administered at 20mg/m 2/d orally from day -7 to day -3. Detailed dosage of the Bu/Flu/Cy comprised of fludarabine 30 mg/m 2/d IV Qd, day -4 to -2, busulfan 3.2 mg/kg/d IV Q6h, day -7 to -5, and cyclophosphamide 40mg/kg/d IV Qd, day -3 to day -2. Either rabbit ATG or porcine ATG was administered at 2.5mg/kg/d IV Qd, day -4 to -1, or 20mg/kg/d IV Qd, day -3 to -1 for GVHD prophylaxis.
Results
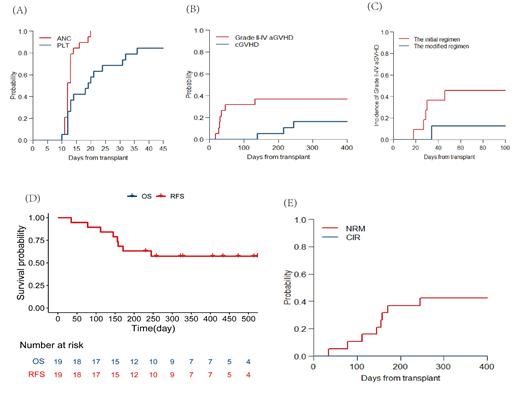
19 patients were enrolled in the trial with a median follow-up time of 258 (range, 35-544) days. Grade 3/4 adverse events included hematological events, hypertension, infections, allergy, and increased amylase. The 30-day cumulative incidence of neutrophil and platelet engraftment was 100% and 68% (Figure 1A).
Acute GVHD occurred in 9 of 19 patients (grade I, n=2; grade II, n=1; grade III, n =4; grade IV, n =2). The cumulative incidence of grade II to IV acute GVHD events at 100 days was 31% (95% confidence interval (CI], 12-53) among all patients, and 45% (95% CI, 15-72), and 13% (95% CI, 0-45) for the initial regimen group and the modified regimen group, respectively (p=0.28, Figure 1B, C). While 1 patient in the modified regimen group occurred diarrhea and was clinically diagnosed with grade III acute GVHD (late-onset) at 132 days post-HSCT. The cumulative incidence of chronic GVHD at 1 year was 37% (95% CI, 16-58).
For the entire cohort, OS and RFS at 6 months was 63% (95% CI, 45-89) and 63% (95% CI, 45-89), and the NRM and CIR at 6 months was 37% (95% CI, 16-58) and 0, respectively (Figure 1D, E). All 8 deaths were attributed to transplant-related complications, including 4 patients from aGVHD, 2 patients from infection, 1 from transplant-associated thrombotic microangiopathy (TA-TMA) and 1 from bloody pleural effusion. Here it should be pointed out that the patient with bloody pleural effusion couldn't exclude the probability of tumor invasion because he had previous esophageal carcinoma.
Conclusion
In conclusion, although the hematological and molecular recurrence rate of the venetoclax combined with the decitabine conditioning regimen is significantly decreased, the high incidence of severe aGVHD and NRM limits its clinical application. It still needs further exploration to optimize the conditioning regimen for high-risk and elderly patients with myeloid neoplasms.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal